In association with TUD excellence cluster Physics of Life (PoL), the Laboratory MST and the Competence Center BIOLAS offer research at the intersection of laser-optical system engineering and cell biology / biomedicine. This research field addresses optogenetics, where the activity of transgenic cells is controlled by means of light-sensitive trans-membrane proteins. Our aim is to study the genesis and termination of light-induced self-sustaining spiral excitation waves in cardiomyocyte networks and organoids by means of means of optogenetic light stimulation and optical read-out in-vitro.
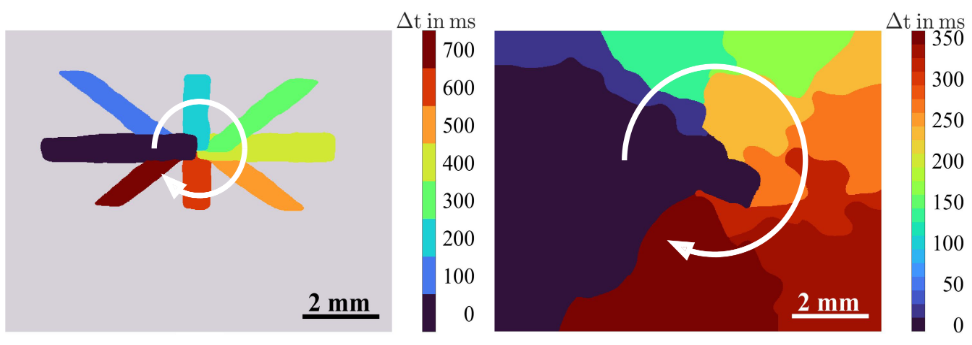
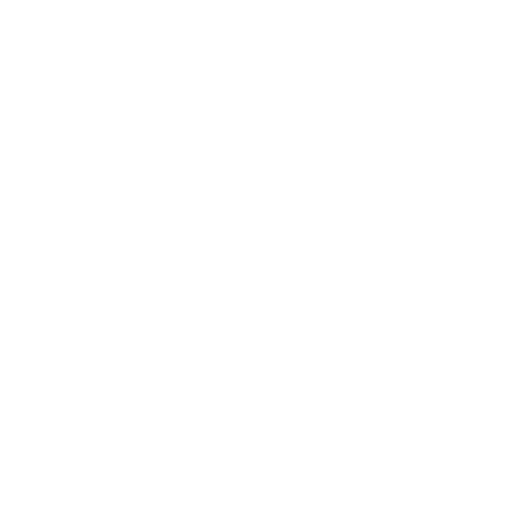
A central aspect of cardiac dysfunction is the disruption of the normal cardiac rhythm. Such disturbances are associated with characteristic spatiotemporal contraction patterns in the cardiac tissue. Understanding the dynamics of pathological contraction patterns is crucial for the development of targeted therapeutic strategies. In this context, optogenetics has emerged as a powerful tool. Optogenetics is a set of methods for the control of the activity of genetically altered cells expressing light-sensitive membrane ion channels. Optical stimulation approaches offer significant advantages over conventional electrical stimulation – e.g., higher spatiotemporal precision. We set up an optical system, combining SLM-based holographic stimulation of human induced stem cell-derived cardiomyocyte monolayers with speckle-based label-free imaging of excitation waves. This enables the dye-free investigation of cardiac dynamics – e.g., by the optical induction of pathological rotating contraction patterns via defined stimulation. Utilizing a method, we developed for reconstructing wavefront characteristics based on sparsely sampled cardiac activity, we were able to realize an all-optical real-time closed-loop control over excitation wavefronts. This allows for the feedback-controlled investigation of cardiac wavefront interactions, possibly improving our understanding of arrhythmia mechanisms and supporting the investigation of optical cardioversion strategies. Future work will extend these imaging and control strategies towards tissue slices or organoids to investigate three-dimensional cardiac phenomena.
References
- R. Wendland, F. Schmieder, M. A. Sikandar, W. H. Zimmermann, L. Büttner, O. Bergmann, J. W. Czarske, Label-Free Microscopy for Optogenetic Investigations of Arrhythmia in Human Cardiomyocyte Networks Expressing Chrimson, European Conferences on Biomedical Optics, SPIE, OPTICA – Munich, June 2025.
- L. Buettner, R. Wendland, F. Schmieder, M. Sikandar, W.-H. Zimmermann, O. Bergmann, J.W. Czarske, “Label-Free Sensing and Real-Time Holographic Optogenetic Control of Cardiac Excitation Wavefronts”, presentation FM1B.2, Frontiers in Optics, 26-30 Oct. 2025, Denver, Colorado, USA
- F. Schmieder, R. Habibey, J. Striebel, L. Büttner, J. Czarske, V. Busskamp. “Tracking connectivity maps in human stem cell-derived neuronal networks by holographic optogenetics” Life Science Alliance, 2022; 5(7):e202101268. https://doi.org/10.26508/lsa.202101268.
- F. Schmieder, L. Büttner, T. Hanitzsch, V. Busskamp, J. W. Czarske. “Two-Wavelength Computational Holography for Aberration-Corrected Simultaneous Optogenetic Stimulation and Inhibition of In Vitro Biological Samples” Applied Sciences, 2022; 12(5):2283. https://doi.org/10.3390/app12052283